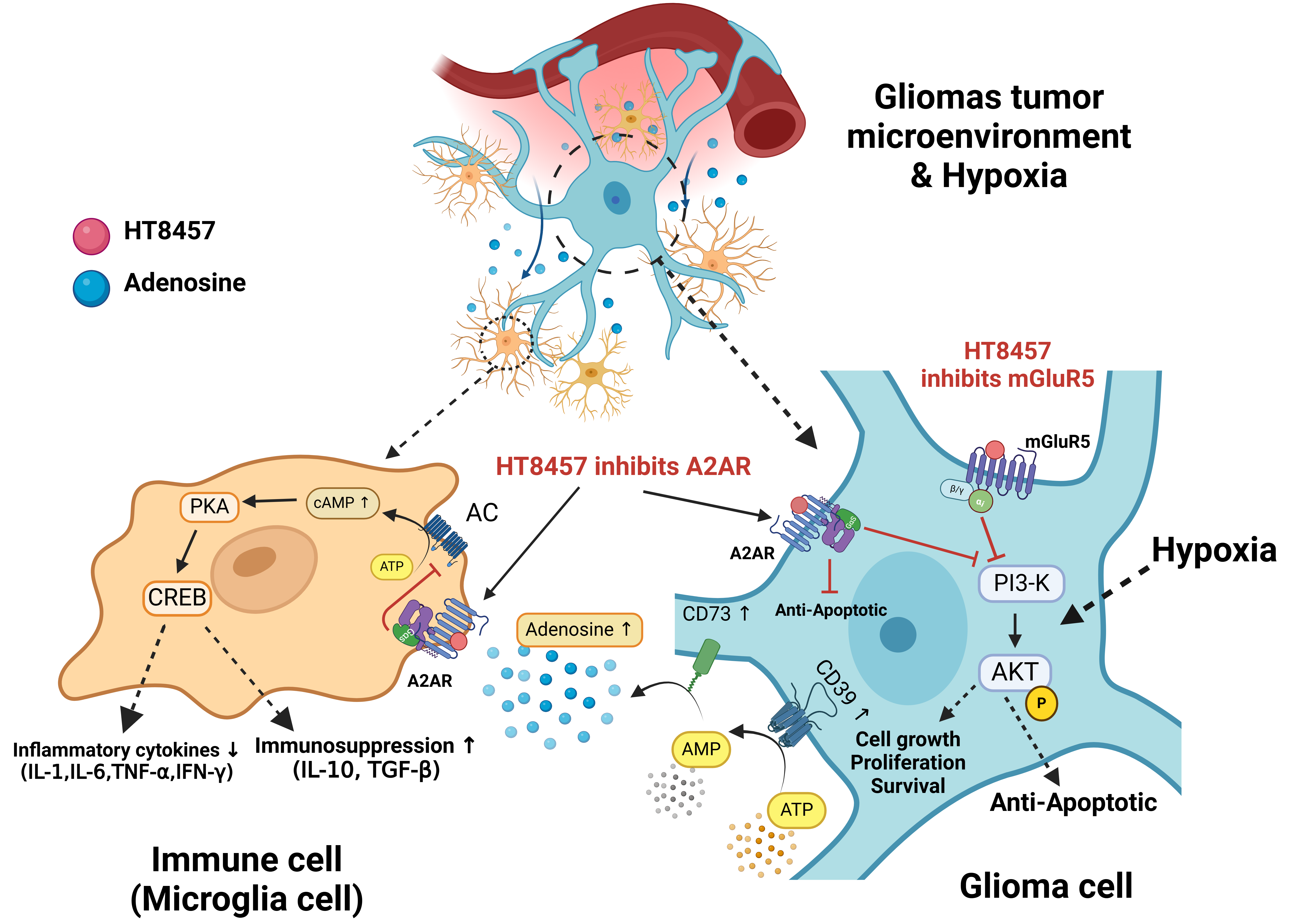
Glioblastomas (GBMs), the most aggressive primary brain tumours, are currently untreatable, with less than 5% of patients surviving beyond five years. Current therapies, including surgery, radiation, and chemotherapy, offer limited efficacy due to tumour resistance, immune evasion and tumour microenvironment constraints such as hypoxia. Innovative approaches are urgently needed to address these challenges. Hado Therapeutics introduces HT8457, a first-in-class therapeutic designed to disrupt GBM progression through dual-target inhibition. HT8457S inhibits Adenosine 2A receptors (A2ARs) on immune cells in the tumour microenvironment to enhance their activity against cancer cells. It also inhibits the mGlu5 receptors (mGluR5) and A2ARs in glioblastoma cells, leading to cell death. The illustration demonstrates HT8457's innovative "Double Tap" mechanism, emphasising its dual-action inhibition of A2ARs and mGluR5 in the glioblastoma microenvironment. This synergy underpins the project’s transformative potential and its ability to redefine therapeutic strategies for glioblastoma.
2. Parkinson's Disease
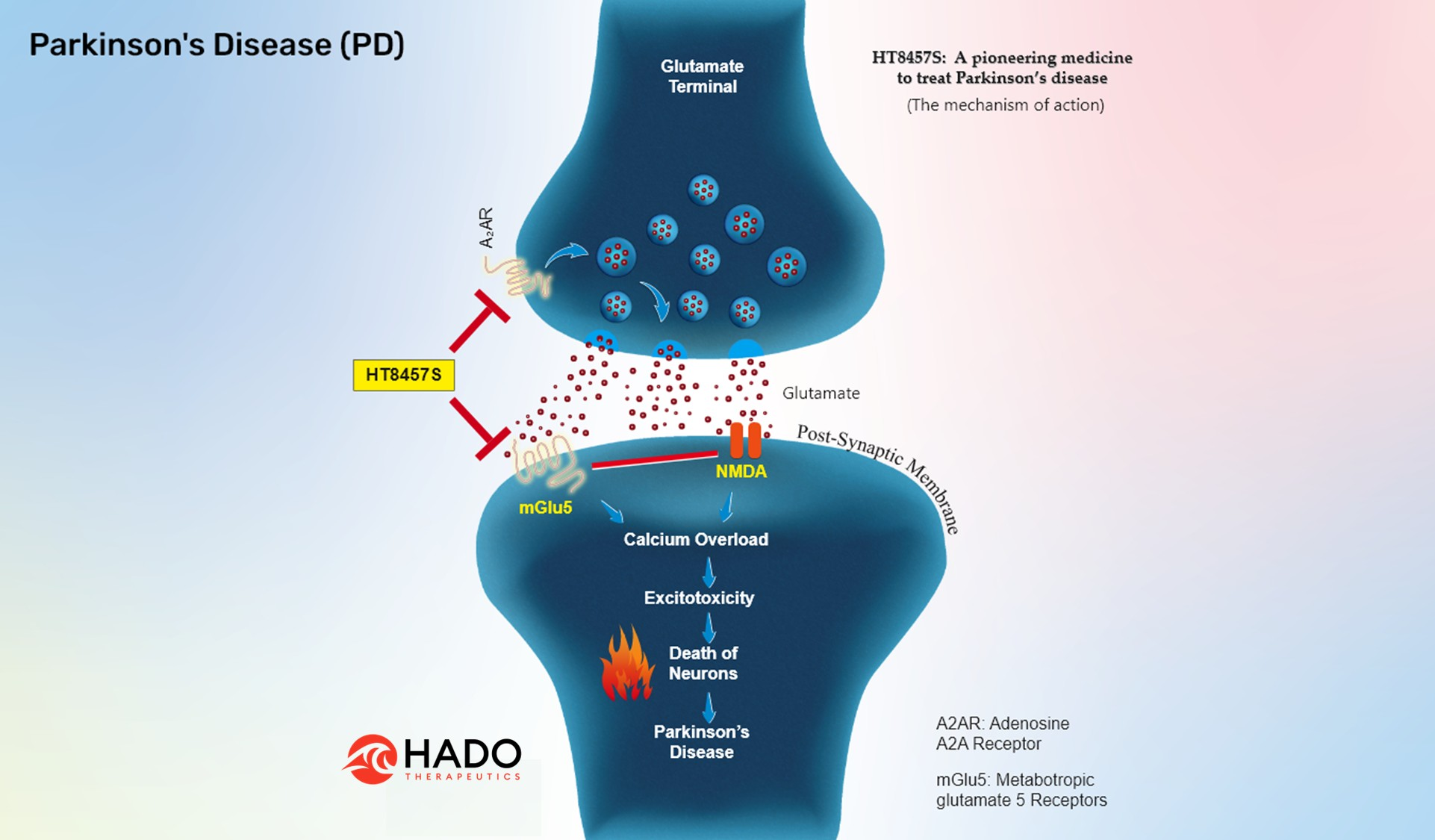
All the available treatments offer symptomatic relief and aim to maintain quality of life. Currently there is no medicine available to cure Parkinson's disease (PD). Hado offers first-in-class molecule, which is currently in an experimental stage. Our strategy uses a small molecule to prevent the death of neurons in the brain by inhibiting two molecular targets within the disease pathway. In a living body, the death of neurons involves multiple targets and inhibiting a single target is a convenient approach for the discovery process but does not stop neurodegeneration. Such strategies have not been effectively translated to the clinic. Our approach to enhancing clinical success is interrupting multiple disease pathways simultaneously, enabling patients' recovery. We use a knowledge-based approach in discovering such molecules as disease-modifying drugs. One of such dual-target inhibitors is HT8457S, which inhibits both 'metabotropic glutamate' mGlu5 receptors (mGluR5) and adenosine A2A receptors (A2AR). Excessive activation of both these receptors are known to cause neurotoxicity and neurodegeneration. By inhibiting A2A and mGlu5 at the same time, we offer the first disease-modifying small molecule for PD treatment, enabling us to contribute a new medicine with a novel treatment approach.
Mechanism of action of HT8457S
Activation of Adenosine (A2A) Receptors regulate glutamate release from presynaptic terminals. The released glutamate will bind to and activate glutamate receptors such as NMDAR and mGluR5 receptors on postsynaptic membranes, enabling calcium entry as well as calcium release from intracellular stores in the postsynaptic neurons. On the other hand, activation of mGluR5 at postsynaptic membranes enhances NMDAR function via signaling proteins.
Thus, upregulation or excessive activation of A2AR at the presynaptic terminals can eventually result in neurotoxicity by releasing glutamate and switching on mGluR5 and NMDAR signaling at the postsynaptic membranes. Furthermore, inhibiting mGluR5 receptors reduces calcium release and NMDA receptor-mediated signaling, with increased efficiency of protecting synapses and neurons.
HT8457S is our first-in-class compound that aims to generate synergetic drug response by simultaneously inhibiting adenosine A2A and mGlu5 receptors to prevent the death of neurons, offering disease-modifying medicine to treat PD patients.